Category: Blog
-
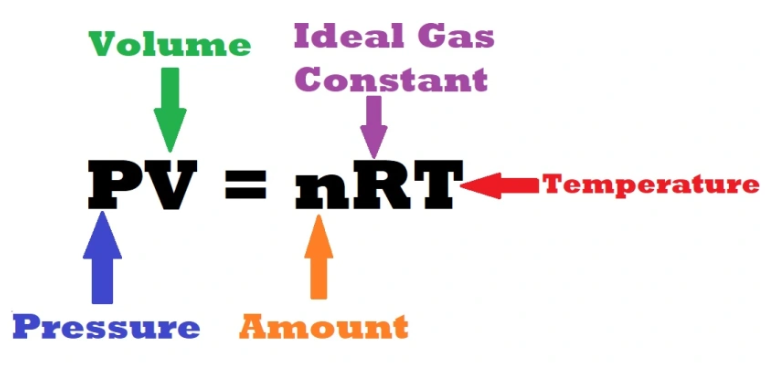
Ideal Gas Law
Ideal Gas Law In 1834 Benoit Paul Émile Clapeyron a French engineer and physicist stated the ideal gas law, which was a result of combination of the Boyle’s Law, Charles’s law, Avogadro’s law, and Gay-Lussac’s law. The Ideal Gas Law is a fundamental equation in chemistry and physics that describes the relationship between the Temperature…
-
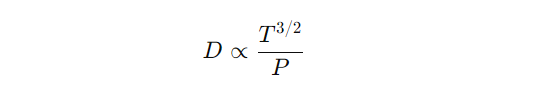
Temperature and Pressure Effect on diffusivity
Temperature and Pressure Effect on diffusivity Short Note The diffusivity of a substance in a fluid, whether it is a gas or a liquid, varies with temperature and pressure. These variations are important in understanding and modeling diffusion processes in different environments, such as chemical reactors, atmospheric science, and biological systems. 1. Temperature Dependence of…
-
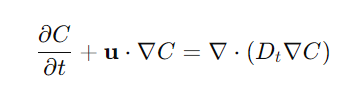
Eddy Diffusion/Forced Diffusion
Eddy Diffusion/Forced Diffusion Eddy diffusion in mass transfer refers to the turbulent transport of mass within a fluid due to the chaotic and random motion of eddies in the flow. For Mass Transfer this type of diffusion is significantly more effective than molecular diffusion, especially in turbulent flows where it dominates the MT aspect leading…
-
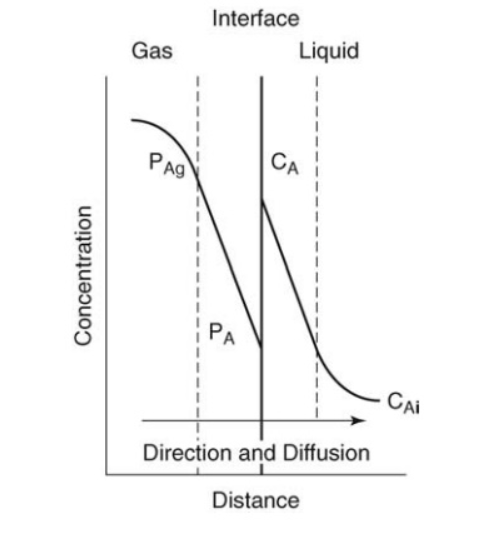
Interphase Mass Transfer
Interphase Mass Transfer Interphase Mass Transfer operation refers to the movement of a substance (mass) from one phase to another phase. Phases Phase refers to state of matter which might be Solid, Liquid and Gas and transfer of matter can be from Gas to Liquid, Liquid to Gas or dissolution of Solid in Gas. Interphase…
-
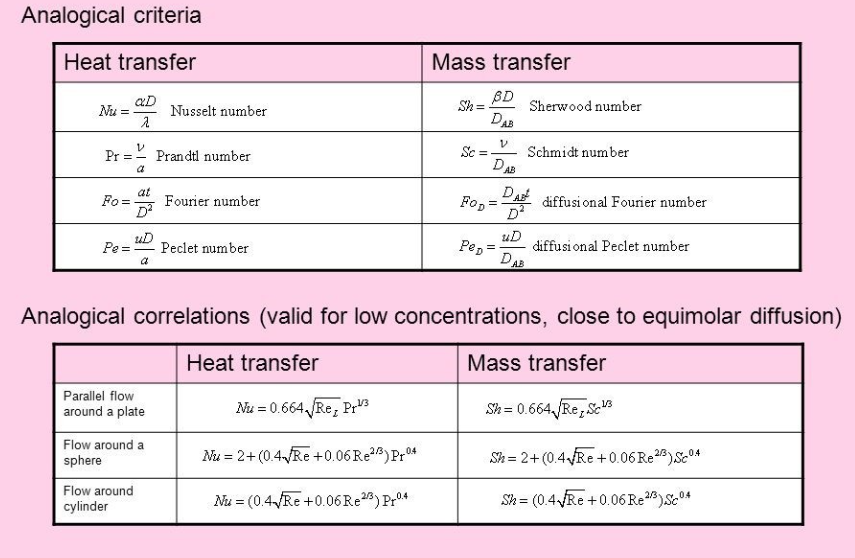
Analogies between Heat and Mass Transfer
Analogies between Heat and Mass Transfer Diffusion – Fick’s law (J = – E dc/dx ) Conduction – Fourier’s law (q = – k dT) Reynolds Analogy | Chilton-Colburn Analogy | Theories of Mass Transfer Important Points: Reynolds and Chilton-Colburn Analogy does not relate K to D (Diffusivity Coefficient) but related K to Heat and Momentum…