Interphase Mass Transfer
Interphase Mass Transfer operation refers to the movement of a substance (mass) from one phase to another phase.
Phases
Phase refers to state of matter which might be Solid, Liquid and Gas and transfer of matter can be from Gas to Liquid, Liquid to Gas or dissolution of Solid in Gas.
Interphase
Interphase is the imaginary boundary separating the two phases, and master transfer is significantly affected by its characteristics.
Driving Force
Driving force for Interphase mass transfer is typically difference in concentration i.e., Concentration Gradient. (Solute moves from a region of higher concentration to a region of lower concentration )
Mass Transfer Coefficient
The rate of Mass Transfer is characterized by a parameter known as Mass Transfer Coefficient, which quantifies rate of mass transfer per unit area per unit concentration difference between the phases. It is influenced by certain conditions such as properties of substance involves, nature of phases, and temperature and pressure of the system.
Two Resistance Film Theory
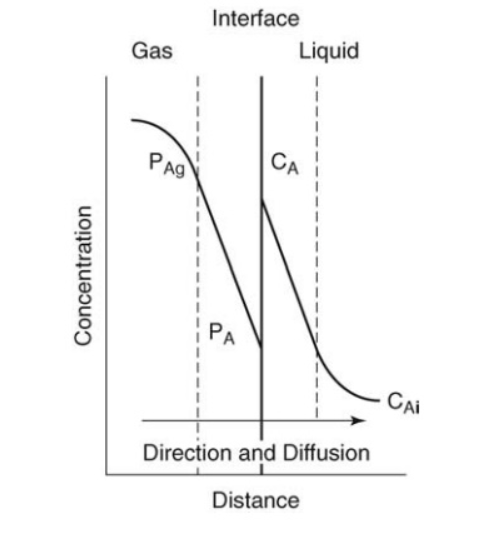
Interphase MT is divided into three main steps:
- Transfer of mass from bulk phase 1 to interphase
- Transfer of mass within interphase
- Transfer of mass from interphase to Bulk phase 2
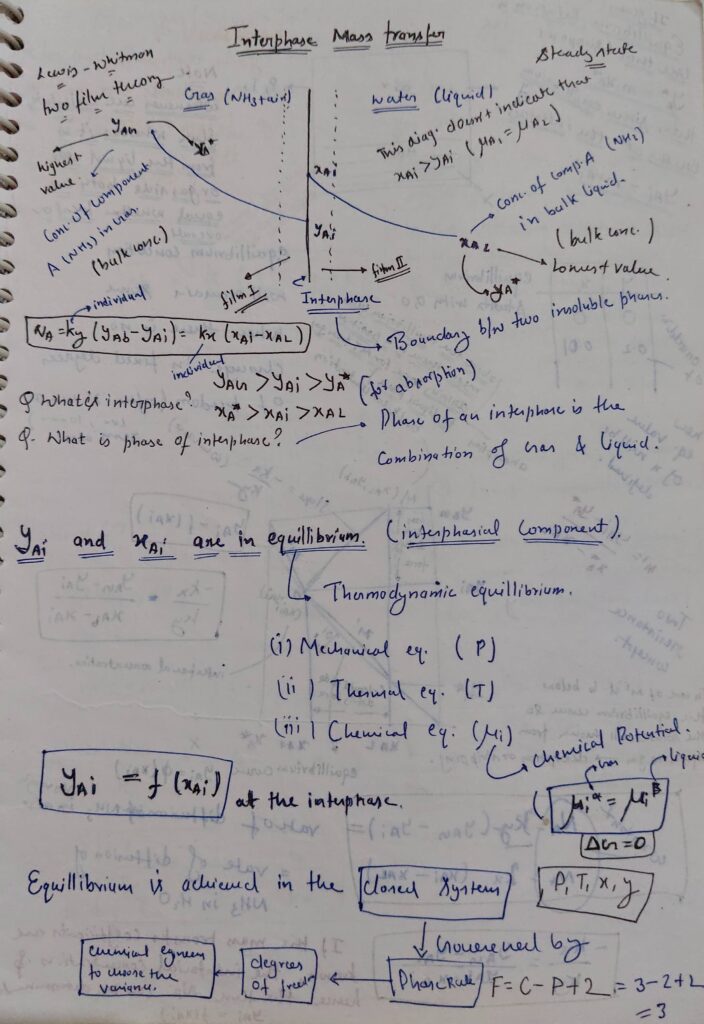
Note:
Concentration of solute in both the phases at interphase is always in equilibrium i.e., for Interphase y = f(x), where y and x are interface concentration in respective phases.
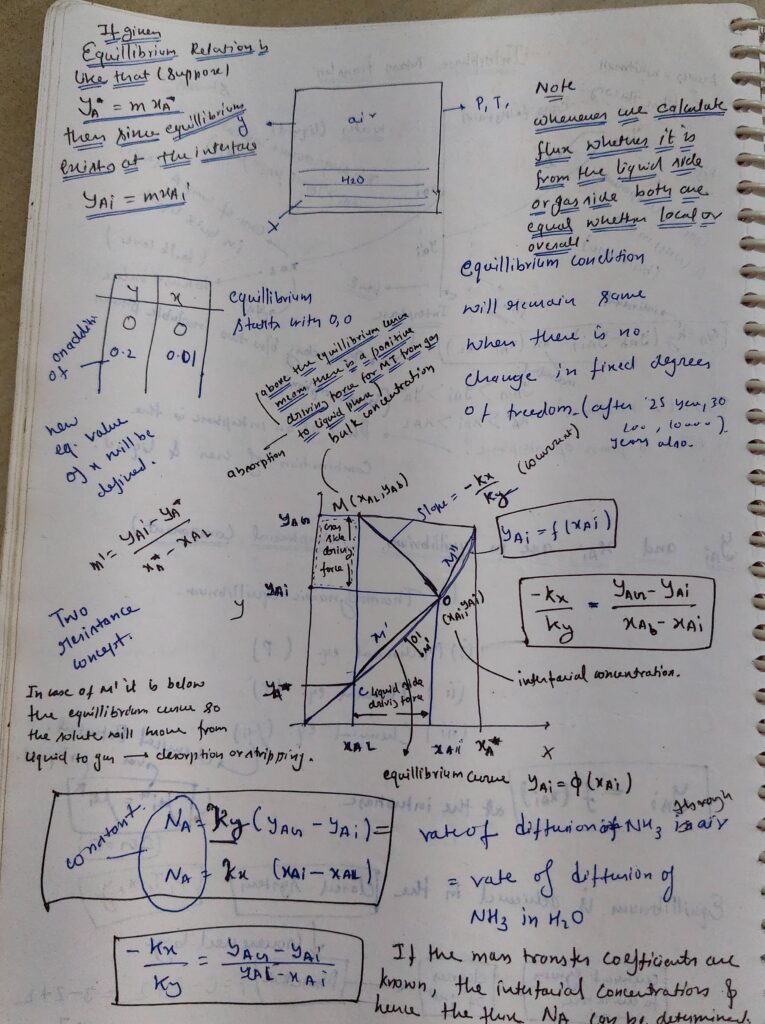

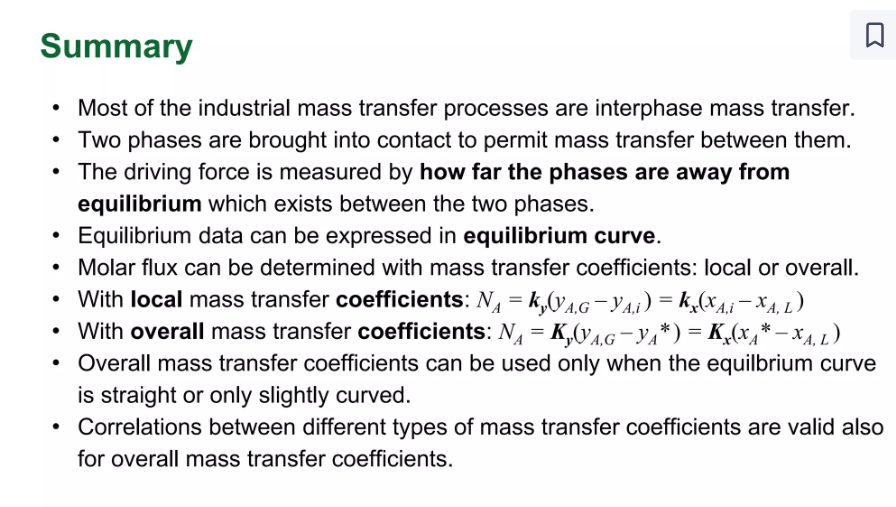
Analogies between Heat and mass Transfer
Click to Compress your Image – Image Compressor | Free | No limit | Upto 95% compression

Leave a Reply
You must be logged in to post a comment.