Temperature and Pressure Effect on diffusivity
Short Note

The diffusivity of a substance in a fluid, whether it is a gas or a liquid, varies with temperature and pressure. These variations are important in understanding and modeling diffusion processes in different environments, such as chemical reactors, atmospheric science, and biological systems.
1. Temperature Dependence of Diffusivity:
Gases
- The diffusivity of gases generally increases with temperature. This is because as temperature increases, the kinetic energy of the molecules increases, leading to more frequent and energetic collisions that facilitate diffusion.
- The relationship between temperature and diffusivity in gases can often be approximated by the equation:
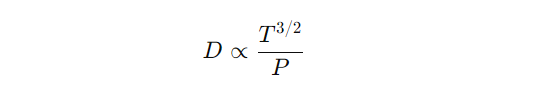
- This indicates that diffusivity increases with the square root of the temperature.
Liquids
The diffusivity in liquids also increases with temperature, but the relationship is more complex due to the stronger intermolecular forces in liquids compared to gases. Typically, the temperature dependence is described by an Arrhenius-type equation

This suggests that diffusivity increases exponentially with temperature.
2. Pressure Dependence of Diffusivity:
Gases
- In gases, diffusivity decreases with increasing pressure. Higher pressure leads to more densely packed molecules, which means the mean free path (the average distance a molecule travels before colliding with another molecule) is reduced. This results in slower diffusion.
- The relationship can be simplified as:
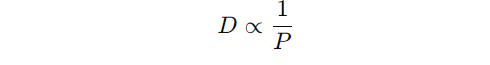
- indicating that diffusivity is inversely proportional to pressure at a constant temperature.
Liquid
- The effect of pressure on diffusivity in liquids is generally less significant compared to gases, especially at moderate pressures. However, at very high pressures, diffusivity can decrease as the liquid becomes denser, reducing the mobility of molecules.
- In many cases, the change in diffusivity with pressure in liquids can be neglected unless dealing with extreme conditions.
Combined Effects:
- Gases: The combined effect of temperature and pressure on gas diffusivity is often represented as
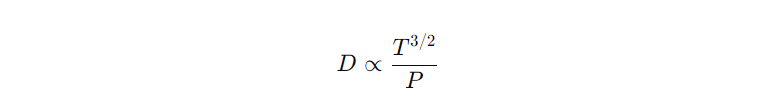
- This shows that increasing temperature increases diffusivity, while increasing pressure decreases it.
- Liquids: For liquids, the combined effects are more complex and typically require empirical correlations or detailed models that account for both temperature and pressure influences.
In summary Temperature and Pressure Effect on diffusivity is as: diffusivity increases with temperature and typically decreases with pressure, with the exact relationship depending on whether the diffusion is occurring in a gas or a liquid.

Leave a Reply
You must be logged in to post a comment.